This is the 3rd article in the BioPharma Domain Series.
The pre-clinical phase in the field of biopharmaceuticals is a crucial stage that sets the foundation for the development of new drugs and therapies. This phase involves a series of rigorous steps aimed at evaluating the safety, efficacy, and potential risks associated with a candidate drug before it progresses to clinical trials in humans. Here is an elaborate overview of the different stages involved in the pre-clinical phase:

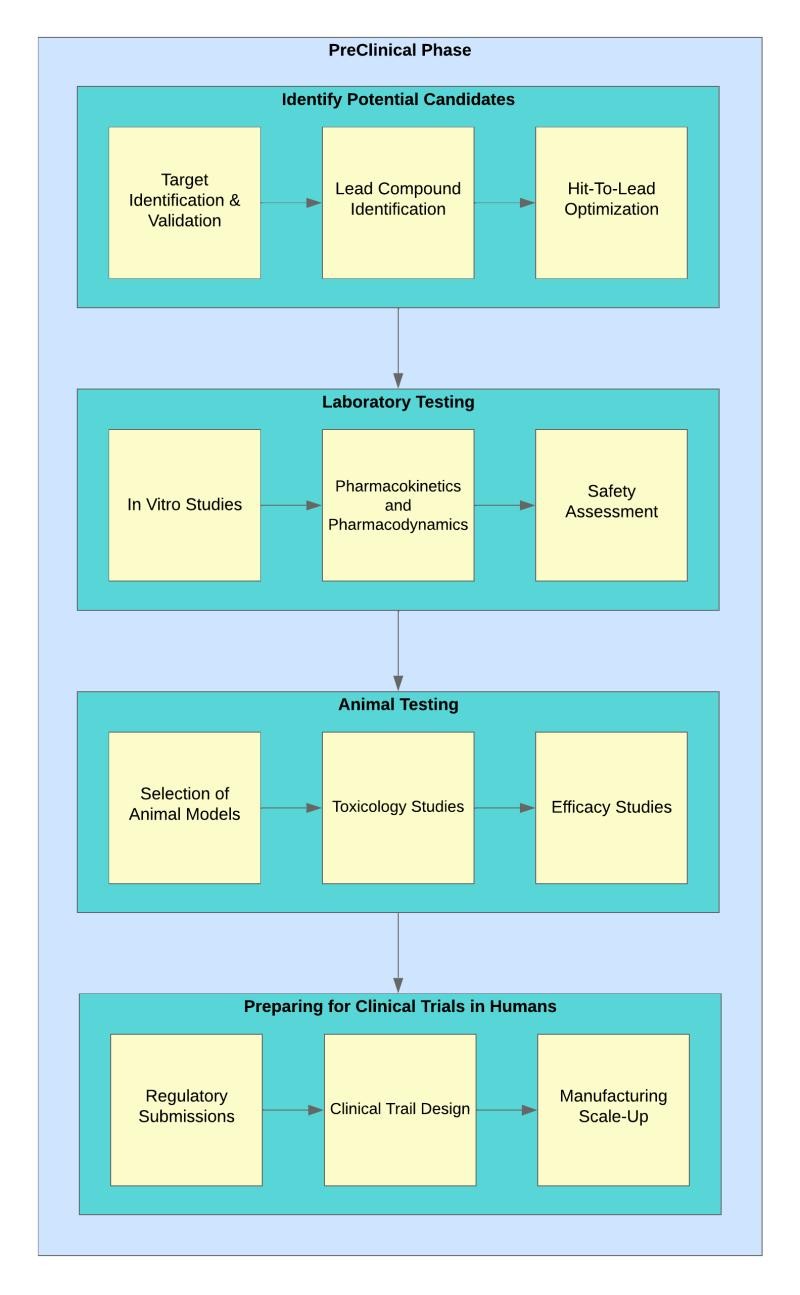
Identifying Potential Candidates:
1. Target Identification and Validation: — At the beginning of drug discovery, researchers identify specific biological targets associated with a particular disease. These targets could be proteins, enzymes, or other molecules that play a key role in the disease process. — Validating these targets involves confirming that modulating them will have a therapeutic effect on the disease. This is often done through various experimental approaches, including genetic studies and biomarker analysis.
2. Lead Compound Identification: — Once validated targets are identified, researchers embark on finding lead compounds that can interact with these targets. This is often done through high-throughput screening, where large libraries of chemical compounds are tested for their ability to bind to the target. — Computational methods, such as molecular modeling and virtual screening, also play a significant role in predicting and optimizing interactions between lead compounds and targets.
3. Hit-to-Lead Optimization: — The identified lead compounds undergo optimization to improve their drug-like properties. Medicinal chemists work on modifying the chemical structure to enhance aspects like bioavailability, stability, and specificity. — Structure-activity relationship (SAR) studies guide the optimization process, ensuring that changes made to the compound positively impact its pharmacological profile.
Laboratory Testing:
1. In vitro Studies:
- In the laboratory, lead compounds undergo extensive testing using cell cultures and tissue samples. These studies help understand how the compounds interact with cells and whether they elicit the desired therapeutic effects.
- Researchers investigate the compound’s mechanism of action, potential off-target effects, and early indications of toxicity.
2. Pharmacokinetics and Pharmacodynamics:
- Understanding how the body processes the drug (pharmacokinetics) and its effects on the body (pharmacodynamics) is critical for further development.
- Pharmacokinetic studies involve tracking the absorption, distribution, metabolism, and excretion of the drug. Pharmacodynamic studies assess the drug’s impact on physiological processes.
3. Safety Assessments:
- Safety studies are conducted to identify any potential adverse effects the drug may have on organs, tissues, or overall physiological functions.
- These studies include cytotoxicity assays, genotoxicity assessments, and other tests to ensure that the drug is safe for progression to in vivo studies.
Animal Testing:
1. Selection of Animal Models:
- Choosing appropriate animal models is crucial for predicting how the drug will behave in humans. Animal models should replicate the relevant aspects of human physiology and disease.
- The choice of models depends on the specific disease being targeted and the expected pharmacological effects of the drug.
2. Toxicology Studies:
- Administering the drug to animals in various doses helps determine its safety profile and potential toxicity. This involves exposing animals to escalating doses of the drug to identify the maximum tolerated dose (MTD).
- Toxicology studies provide crucial information for establishing a safe starting dose for human clinical trials.
3. Efficacy Studies:
- Animal models are employed to assess the drug’s efficacy in treating the targeted disease. Positive results in animals provide early evidence of the drug’s potential effectiveness in humans.
- Efficacy studies help researchers understand the therapeutic impact of the drug and guide the design of human clinical trials.
Preparing for Clinical Trials in Humans:
1. Regulatory Submissions:
- Researchers compile data from pre-clinical studies to submit Investigational New Drug (IND) applications to regulatory authorities, such as the FDA.
- Regulatory agencies rigorously review the pre-clinical data to ensure that the potential benefits of the drug outweigh the risks, paving the way for human trials.
2. Clinical Trial Design:
- Based on pre-clinical findings, researchers design the parameters for clinical trials, including dosage, patient population, and trial duration.
- Phase I trials focus on safety, Phase II on efficacy and side effects, and Phase III involves larger populations to confirm effectiveness and monitor rare side effects.
3. Manufacturing Scale-Up:
- Preparing for clinical trials requires the production of larger quantities of the drug. Manufacturing processes are scaled up to meet the demands of human trials while maintaining the quality and consistency of the drug product.
- Good Manufacturing Practice (GMP) standards are followed to ensure the production of high-quality investigational medicinal products.
In conclusion, the pre-clinical phase is a complex and multifaceted process that involves in-depth research, laboratory testing, animal studies, and meticulous preparation for human clinical trials. It serves as a critical bridge between initial drug discovery and the evaluation of safety and efficacy in human subjects, ultimately shaping the trajectory of drug development in the biopharmaceutical industry.